Explain the Difference Between End Point and Equivalence Point
The end point is when you see the change in the indicator the changing color of the indicator and of course you want that to be Ah what youre really trying to see with that. Equivalence point can be multiple in number during titration but the endpoint is the one point and does not occur frequently.
Difference Between Endpoint And Equivalence Point Difference Between
Explain the difference between an endpoint and an equivalence point in a titration.
. Explain how the pH at the equivalence point varies as the strength of the acid increases. So the difference between the equivalence point of dehydration and the endpoint the easiest way to understand it is that the equivalence point is just a theoretical point that you would like figure out on your paper in calculations theoretical and then the endpoint is something that you would actually observed in the lab. Main Differences Between Endpoint and Equivalence Point.
The NaOH solution used during the titration will be standardized by the lab prep staff before it is used in the experiment. 4 rows A point of equivalence in a titration refers to a point at which the added titrant is. Label the curve with the following items.
Remember that acid strength depends upon Ka not upon analytical concentration. The main difference between equivalence point and endpoint is that equivalence point is the precise finishing point where the chemical reaction usually comes to end whereas endpoint is the limit where the alteration in color happens in the arrangement. The key difference between equivalence point and endpoint is that the equivalence point in a titration is the point at which the added titrant is chemically equivalent completely to the analyte in the sample whereas the endpoint is the point where the indicator changes its colour.
1 Explain the difference between equivalence point and end point. Explain the difference between the end point and the equivalence point in a titration. So at the equivalence point its the point when you calculate that.
First equivalence point second equivalence point first halfway point halfway between first and second equivalence points. There will be more than one equivalence point for polyprotic acids and bases with more than 1 x OH- ions. Consider the titration curve for a diprotic acid.
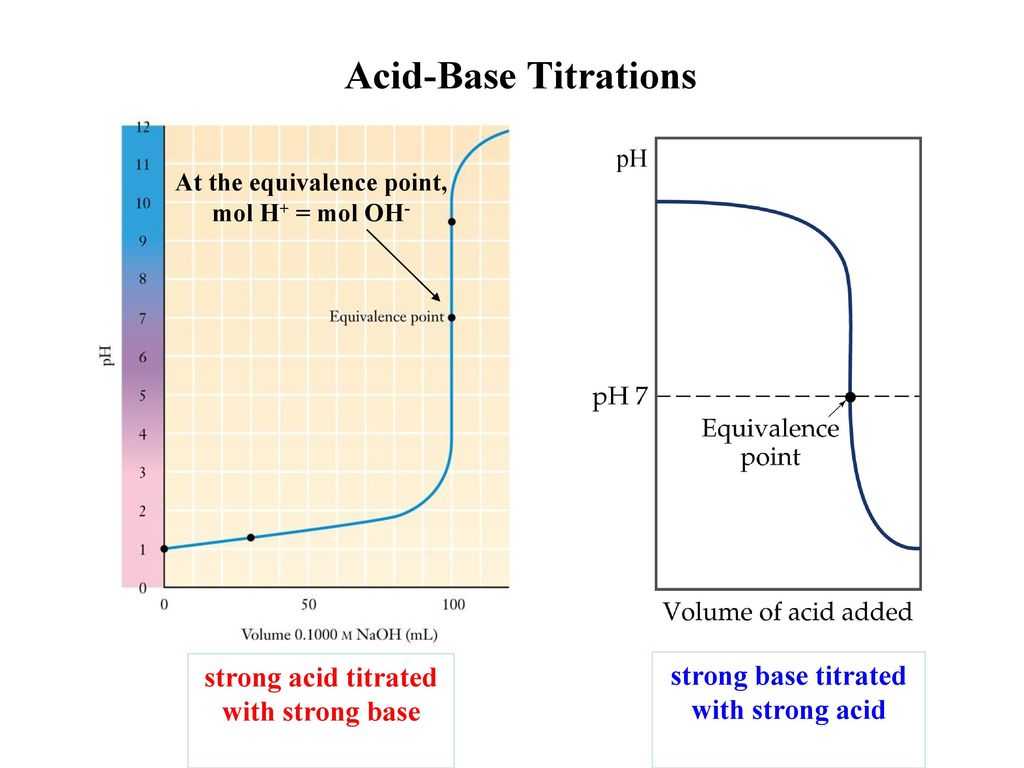
Endpoint and equivalence can occur at the same time if the pH of the titrant corresponds to the pH at equivalence point. 2 10 20 30 Volume of NaOH added mL a. Endpoint and stoichiometric point in common equivalence point are always different from each other.
The key difference between half equivalence point and equivalence point is that half equivalence point is the midpoint between the starting point and the equivalence point of a particular titration whereas equivalence point is where the chemical reaction ends. Explain the difference between equivalence point and end point. Add your answer and earn points.
Why is it important to calibrate the pH meter. The key difference between endpoint and stoichiometric point is that endpoint comes just after the stoichiometric point whereas stoichiometric point is the most accurate point at which the neutralization completes. 12 10 00 pH 6 4.
Titrations are analytical techniques in chemistry that are important in determining the unknown. Explain the difference between an endpoint and equivalence point in a titration - 14819792 slimshady45 slimshady45 02182020 Chemistry High School answered Explain the difference between an endpoint and equivalence point in a titration 1 See answer slimshady45 is waiting for your help. Equivalence point is the point where equal number of moles of acid and the number of moles of base that have been mixed together are equal.
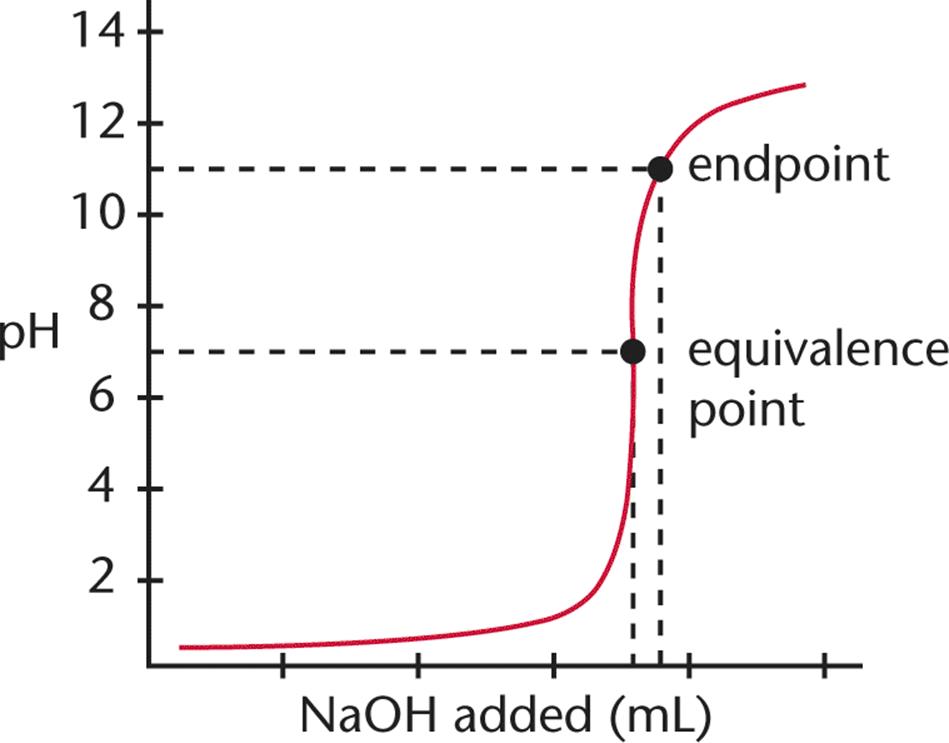
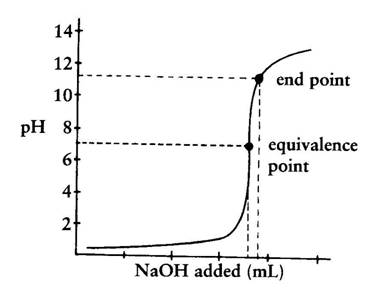
Explain the difference between the end point and the equivalence point in a titration. The endpoint indicates that the equivalence point has been reached. Chemistry questions and answers.
Equivalence point is the actual point where the reaction has just been completed and refers to the stoichiometric quantities of reactants eg when 1 mole HCl is added to 1 mole of NaOH. Equivalence point means that the titrant has reacted fully with the analyte whereas the endpoint signals the completion of titration. So end point is the point shown by an indicator.
Endpoints can be reached with or after the point of equivalence. The main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical reaction ends whereas end point is. Your answer should be based upon the proton transfer reaction that is important at the equivalence point.
End point is the point at which the indicator being used in such a reaction changes colour. Here we have a 1 to 1 reaction between the ammonia and HCL and so the number of moles of HCL in the uh in the Thai Trey shin flask will be equal to the number of moles of ammonia at the equivalents point. Endpoint is the point in the titration where the indicator changes its color.
Endpoint Vs Equivalence Point What S The Main Difference Psiberg
Endpoint Vs Equivalence Point What S The Main Difference Psiberg

Difference Between Half Equivalence Point And Equivalence Point Compare The Difference Between Similar Terms
Difference Between Equivalence Point And Endpoint Definition Properties Examples
Acid Base Titrations End Point And Equivalence Point Ppt Download

Equivalence Point Labster Theory
Difference Between Equivalence Point And Endpoint Definition Properties Examples
Difference Between Endpoint And Equivalence Point Difference Between
Difference Between Endpoint And Equivalence Point Difference Between
Endpoint Vs Equivalence Point What S The Main Difference Psiberg
Difference Between Endpoint And Equivalence Point Difference Between
Titration And Buffers Acids And Bases Training Mcat General Chemistry Review

Titration Curves Equivalence Point Article Khan Academy
Difference Between Equivalence Point And Endpoint Definition Properties Examples
Endpoint Vs Equivalence Point What S The Main Difference Psiberg

Difference Between Half Equivalence Point And Equivalence Point Compare The Difference Between Similar Terms
Endpoint Vs Equivalence Point What S The Main Difference Psiberg
Difference Between Endpoint And Equivalence Point Difference Between
Comments
Post a Comment